X-ray Generation from Atoms Quiz
X-ray Generation From Atoms Solution
Neils Bohr numbered the energy levels (n) of hydrogen, with level 1 (n=1) being the ground state, level 2 being the first excited state, and so on. Remember that there is a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is considered to be ionized (or "free"). In that case n approaches infinity.The equation for determining the energy of any state (the nth) is as follows:
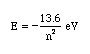
Answer the following questions:

