What is Your Cosmic Connection to the Elements?
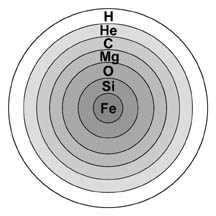
Stars larger than eight times the mass of our Sun begin their lives the same way smaller stars do: by fusing hydrogen into helium. However, a large star burns hotter and faster, fusing all the hydrogen in its core to helium in less than 1 billion years. The star then becomes a red supergiant, similar to a red giant, only larger. Unlike red giants, these red supergiants have enough mass to create greater gravitational pressure, and therefore higher core temperatures. They fuse helium into carbon, carbon and helium into oxygen, and two carbon atoms into magnesium. Through a combination of such processes, successively heavier elements, up to iron, are formed (see Table 1). Each successive process requires a higher temperature (up to 3.3 billion kelvins) and lasts for a shorter amount of time (as short as a few days). The structure of a red supergiant becomes like an onion (see Figure 3), with different elements being fused at different temperatures in layers around the core. Convection brings the elements near the star's surface, where the strong stellar winds disperse them into space.
|
|||||||||||||||||||||||||||||||||||
| Table 1 - This table shows the nucleosynthesis reactions that occur in successive stages in large stars. The table summarizes the chief reactions and their products (including other elements that are produced in trace amounts), the temperature at which the reaction occurs, and how long it takes to use up the available input fuel. |
|
| Figure 3: The "onion shell" model of a red super giant. |
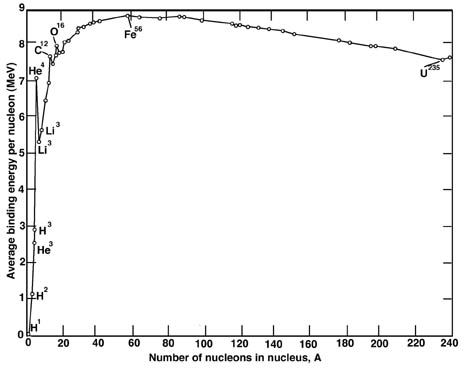
Fusion continues in red supergiants until iron is formed. Unlike the elements before it, iron releases no energy when fused. This is because iron has the most stable nucleus of all the elements. Elements lighter than iron generally emit energy if fused, since they move from a less stable nuclear structure to a more stable one. By contrast, elements heavier than iron emit energy if they undergo fission, that is, by losing nucleons (i.e. protons and/or neutrons). Again, they go from a less stable to a more stable nuclear structure. This is illustrated in greater detail by the "Binding Energy Per Nucleon" chart (Figure 4). The number of nucleons in the nucleus, plotted along the x-axis, is equivalent to the atomic weight of the atom. "Binding energy per nucleon" represents the amount of energy necessary to break the nucleus apart into separate protons and neutrons. The plot shows how this binding energy changes with increasing atomic weight. The stability of the iron nucleus is represented by the fact that it requires the most energy to break apart.
Another reason fusion does not go beyond iron is that the temperatures necessary become so high that the nuclei "melt" before they can fuse. That is, the thermal energy due to the high temperature breaks silicon nuclei into separate helium nuclei. These helium nuclei then combine with elements such as chlorine, argon, potassium, and calcium to make elements from titanium through iron.
Large stars also produce elements heavier than iron via neutron capture. Because of higher temperatures in large stars, the neutrons are supplied from the interaction of helium with neon. This neutron capture process takes place over thousands of years. The abundances of selected elements from iron to zirconium can be attributed to this type of production in large stars. Again, convection and stellar winds help disperse these elements.
Recommended Activity: Cosmic Shuffle