Student Worksheet: The Flame Test
The Flame Test Student Worksheet
Part I
Procedure
- Put on lab apron and safety goggles.
- Add 15 drops of each 0.5M solution to a different clean test tube.
- To clean the wire, dip it into the test tube of 1M of HCl and heat the wire in the hottest part of the flame until no color shows.
- When the platinum wire is clean, dip the wire in the test tube containing a 0.5M solution and hold it in the hottest part of the flame. Record your observation of the color of the flame on the data table.
- Repeat the process of cleaning the platinum wire. Now get ready to test another solution.
- Test all of the solutions and make sure that you record the color of the flame for each element on the Data Table.
- Check your flame colors to known results.
- Fill one clean test tube with 15 drops of one of the 0.5M solutions. The teacher keeps track of what element solution is in this "mystery tube." Repeat the flame test, without telling the students what solution it is. Students must use the information gained from the first part of the experiment to identify the mystery solution.
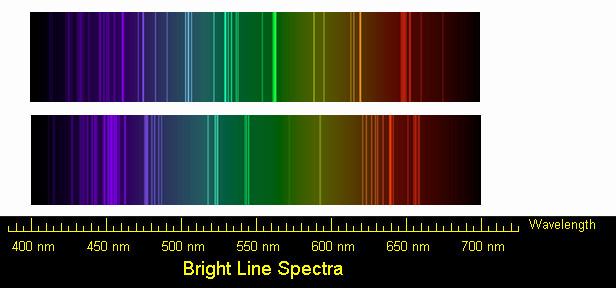
- Use the diffraction grating to observe the color of the flame for the following elements: Sodium, Barium, Copper, and Lithium. The students should be able to see the individual lines making up the light from the flame. This can be tricky! In order for it to work, the room will have to be completely dark (in order to block out other light sources) and the students will have to be close to the flame, holding the diffraction grating up to their eyes. It may be necessary to rotate the diffraction grating in order to see the emission lines. Be patient!
- Record the colors of the elements' emission lines in column three of the Data Table.
- Before leaving the laboratory, wash your hands thoroughly with soap and water.
| Stations | Observed Flame Color |
Color of Emission Lines |
l (m) | n (Hz) | E (J) |
| Calcium (0.5M CaCl) | |||||
| Sodium (0.5M NaCl) | |||||
| Barium (0.5M BaCl) | |||||
| Lithium (0.5M LiCl) | |||||
| Copper (0.5M CuCl) | |||||
| Cesium (0.5MCsCl) |
Think About
|
Part II