Student Worksheet: A Calculation Investigation - Solution
EM Spectrum - A Calculation Investigation Student Worksheet - Solution
From
we can solve for the frequency:
Subsitute this into:
to get
| Electromagnetic Radiation Range |
|||
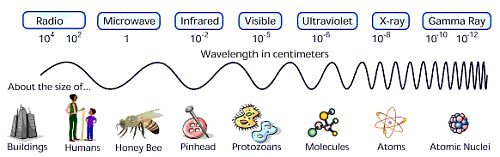
Thought QuestionsStudents should note the inverse relationship between wavelength and frequency: as wavelength increases, frequency decreases OR as wavelength decreases, frequency increases. They should note a similar inverse relationship between wavelength and energy. Students should also note the linear, correlated relationship between frequency and energy: as frequency increases, energy increases. Students might also compare the size of the wavelength of various waves to the sizes of common objects, as illustrated in the above figure. They might also note how small the energies are.
|

