Student Worksheet: EM Spectrum - A Calculation Investigation
You are given the following two equations that express the relationships
between the speed, the
wavelength, the energy and the
frequency of
light:
c = l n
speed = wavelength x frequency
E = hn
energy = Planck's constant x frequency
Answer This!
- Check the equations above and show that the units match on each
side of the equations.
- Manipulate both equations to solve for energy (E) as a function
of wavelength (l) and fundamental constants.
Show each step. Show that the units match on each side of the
resulting equations.
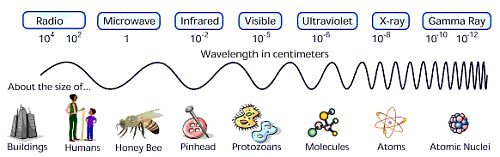
- Given a photon's wavelength, frequency or energy in the chart
below, use the above equations to solve for the other two (in the
units indicated). Use the useful
constants page if you need to. Use the chart of the
electromagnetic spectrum (below the table) to fill in the part of the
electromagnetic radiation range for each row.
|
| Wavelength (m) |
Frequency (Hz) |
Energy (J) |
Electromagnetic
Radiation Range |
| 0.001 |
|
|
|
| |
7.0 x 1013 |
|
|
| 5.0 x 10-7 |
|
|
|
| |
|
2.0 x 10-15 |
|
| |
1.2 x 1022 |
|
|
Thought Questions
In three minutes, summarize what you have learned about light and the
relationship between its energy, frequency and wavelength. Write an
unanswered question you still have. |


