Student Worksheet: Energy Levels in the Atom
Calculate the Energy! Student Worksheet
The equation for determining the energy of any state (the nth) is as follows:
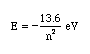
Answer the following questions:
1. Using the above expression, calculate the energy of the first excited state. Your answer will be negative. This signifies that the electron is bound to the atom (as opposed to being a free electron).
2 . Use the above expression to find the energy of the photon released when an electron around a hydrogen atom moves from the 4th to the 2nd level.
3. Now use the above expression to find the energy of the photon released when a free electron is captured to the 2nd level.
4. Use the relationship between a photon's energy and its wavelength to calculate the wavelength of the photon emitted in question 2.
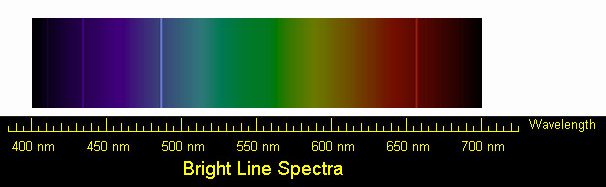
5. Compare the wavelength for this transition with the lab spectrum of hydrogen below.