Understanding the Atom
Understanding the Atom
The nucleus of an atom is surround by electrons that occupy shells, or orbitals of varying energy levels.
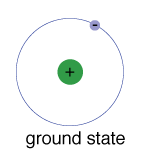
The ground state of an electron, the energy level it normally occupies, is the state of lowest energy for that electron.
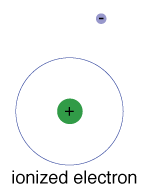
There is also a maximum energy that each electron can have and still be part of its atom. Beyond that energy, the electron is no longer bound to the nucleus of the atom and it is considered to be ionized.
When an electron temporarily occupies an energy state greater than its ground state, it is in an excited state. An electron can become excited if it is given extra energy, such as if it absorbs a photon, or packet, of light, or collides with a nearby atom or particle.
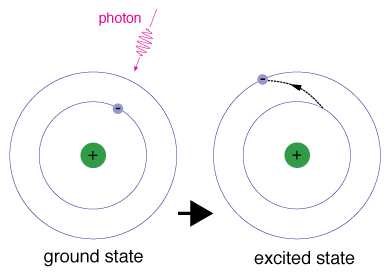
Each orbital has a specific energy associated with it. For an electron to be boosted to an orbital with a higher energy, it must overcome the difference in energy between the orbital it is in, and the orbital to which is is going. This means that it must absorb a photon that contains precisely that amount of energy, or take exactly that amount of energy from another particle in a collision.
Electrons do not stay in excited states for very long - they soon return to their ground states, emitting a photon with the same energy as the one that was absorbed.

Transitions among the various orbitals are unique for each element because the energy levels are uniquely determined by the protons and neutrons in the nucleus. When the electrons of a certain atom return to lower orbitals from excited states, the photons they emit have energies that are characteristic of that kind of atom. This gives each element a unique fingerprint, making it possible to identify the elements present in a container of gas, or even a star.
Updated: November 2013

