Background: Introduction to Spectroscopy
Introduction to Spectroscopy
| Spectroscopy is a complex art - but it can be very useful in helping scientists understand how an object like a black hole, neutron star, or active galaxy is producing light, how fast it is moving, and even what elements it is made of. A spectrum is simply a chart or a graph that shows the intensity of light being emitted over a range of energies. Spectra can be produced for any energy of light - from low-energy radio waves to very high-energy gamma-rays. |
Spectra are complex because each spectrum holds a wide variety of information. For instance, there are many different mechanisms by which an object, like a star, can produce light - or using the technical term for light, electromagnetic radiation. Each of these mechanisms has a characteristic spectrum.
|
Let's look at a spectrum and examine each part of it.
To the right is an X-ray spectrum made using data from the ASCA satellite. It is of a supernova remnant (SNR) - a SNR is a huge cloud of gaseous matter swept up from the explosion of a massive star. The X-axis shows the range of energy of light that is being emitted. The Y-axis of the graph shows the intensity of the light recorded by the instrument from the SNR - - that is, the number of photons of light the SNR is giving off at each energy, multiplied by the sensitivity of the instrument at that energy. We can tell that the light, or radiation, from this SNR is very high energy - if we look at the units of the X-axis - we can see that the photons of light have energys measured in keV, or kilo-electron Volts. A kilo-electron Volt is 1000 electron Volts (eV). This puts is the X-ray range of the electromagnetic spectrum. |
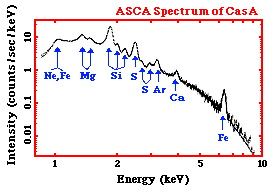 |
The graph shows a decreasing curve, with lots of bumps in it. The curve itself is called a continuum - it represents X-ray photons emitted at all energies continuously. The X-rays that are producing this continuum can be caused by several mechanism that are completely different than those producing the X-rays at the various peaks and bumps on the curve. The peaks and bumps are called line emission. Not only are these two different kind of X-ray emission (continuum and line) produced differently, but they each tell us different things about the source that is emitting them.
The Electromagnetic Spectrum
White light (what we call visible or optical light) can be split up into its colors easily and with a familiar result - the rainbow. All we have to do is use a slit to focus a narrow beam of the light at a prism. This set-up is actually a basic spectrometer.

The resultant rainbow is really a continous spectrum that shows us the different energies light (from red to blue) present in visible light. But the electromagnetic spectrum encompasses more than just optical light - it covers all energies of light extending from low-energy radio waves, to microwaves, to infrared, to optical light, to ultraviolet, to very high-energy X- and gamma-rays.
Line Emission
Instead of using our spectrometer on a light bulb, what if we were to use it to look a tube of gas - for example, hydrogen? We would first need to heat the hydrogen to very high temperatures, or give the atoms of hydrogen energy by running an electric current through the tube. This would cause the gas to glow - to emit radiation. If we looked at the spectrum of light given off by the hydrogen gas with our spectroscope, instead of seeing a continuum of colors, we would just see a few bright lines. Below we see the spectrum, the unique fingerprint of hydrogen.

These bright lines are called emission lines. Remember how we heated the hydrogen to give the atoms energy? By doing that, we excited the electrons in the atom - when the electrons fell back to their ground state, they gave off photons of light at hydrogen's characteristic energies. If we altered the amount or abundance of hydrogen gas we have, we could change the intensity of the lines, that is, their brightness, because more photons would be produced. But we couldn't change their color - no matter how much or how little hydrogen gas was present, the pattern of lines would be the same. Hydrogen's pattern of emission lines is unique to it. The brightness of the emission lines can give us a great deal of information about the abundance of hydrogen present. This is particularly useful in a star, where there are many elements mixed together.
Each element in the periodic table can appear in gaseous form and will each produce a series of bright emission lines unique to that element. The spectrum of hydrogen will not look like the spectrum of helium, or the spectrum of carbon, or of any other element.
Hydrogen:

Helium:

Carbon:

We know that the continuum of the electromagnetic spectrum extends from low-energy radio waves, to microwaves, to infrared, to optical light, to ultraviolet, to X and gamma-rays. In the same way, hydrogen's unique spectrum extends over a range, as do the spectra of the other elements. The above spectra are in the optical range of light. Line emission can actually occur at any energy of light (i.e. visible, UV, etc. ) and with any type of atom, however, not all atoms have line emission at all wavelengths. The difference in energy between levels in the atom is not great enough for the emission to be X-rays in atoms of lighter elements, for example.
Different Graphical Representations of Spectra
Below, you will see the spectrum of the Sun at ultraviolet wavelengths. There are distinct lines (in the top graph) and peaks (in the bottom one) and if you look at the X-axis, you can see what energies they correspond to. For example, we know that helium emits light at a wavelength of 304 angstroms, so if we see a peak at that wavelength, we know that there is helium present.
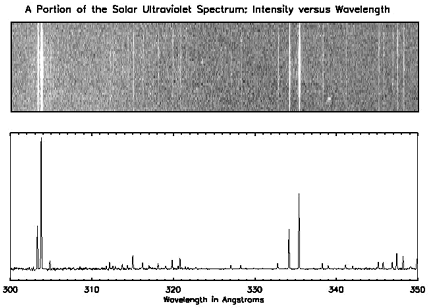
Spectra and Astronomy
Spectral information, particularly from energies of light other than optical, can tell us about material around stars. This material may have been pulled from a companion star by a black hole or a neutron star, where it will form an orbiting disk. Around a compact object (black hole, neutron star), the material in this accretion disk is heated to the point that it gives off X-rays, and the material eventually falls onto the black hole or neutron star. It is by looking at the spectrum of X-rays being emitted by that object and its surrounding disk, that we can learn about the nature of these objects.
Continuum Emission

Just like visible light, with its range of energies from red to blue, X-rays have a continuum, or a range of energies associated with it. X-rays usually range in energy from around 0.5 keV up to around 1000 keV.
Like line emission, continuum X-ray emission involves charged particles. Continuum emission is a result of the acceleration of a population of charged particles. All X-ray sources contain such particles. These particles must be at least partially ionized - their electrons need to be unbound from their nuclei to be free to zip around when they are heated to extreme temperatures. For an electron to radiate X-rays, the gas containing the electron must have extreme conditions, such as temperatures of millions of degrees, super-strong magnetic fields, or the electrons themselves must be moving at nearly the speed of light. Extreme conditions can be found in disks of matter orbiting black holes or in supernova remnants. Strong magnetic fields, like those created in the wake of a supernova explosion, can also accelerate fast moving ions in spirals around the field lines to the point of X-ray emission. Electrons can be accelerated to nearly the speed of light in the shockwave created by a supernova explosion.
There are three mechanisms that will produce a continuum X-ray emission. They are Synchrotron Radiation, Bremsstrahlung, and Compton Scattering. The radiation produced is continuous, and not at the discreet energies of line emission because the populations of electrons have a continuous range of energies, and they can be accelerated through a range of energies, .
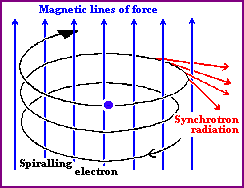
courtesy of University of Hertfordshire | Sychrotron radiation is emitted when a fast electron interacts with a magnetic field. A magnetic field in an area an electron is traveling in will cause the electron to change direction by exerting a force on it perpendicular to the direction the electron is moving. As a result, the electron will be accelerated, causing it to radiate electromagnetic energy. This is called magnetic bremsstrahlung or synchrotron radiation (after radiation observed from particle accelerators by that name). If the electrons and the magnetic field are energetic enough, the emitted radiation can be in the form of X-rays. |
| Bremsstrahlung occurs when an electron passes close to a positive ion, and the strong electric forces cause its trajectory to change. The acceleration of the electron in this way causes it to radiate electromagnetic energy - this radiation is called bremsstrahlung, (literally, from the German meaning 'braking radiation'). Thermal bremsstrahlung occurs in a hot gas, where many electrons are stripped from their nuclei, leaving a population of electrons and positive ions. If the gas is hot enough (millions of degrees Kelvin), this kind of radiation will primarily take the form of X-rays. | 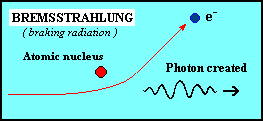
courtesy of University of Hertfordshire |

courtesy of University of Hertfordshire | Comptonization is when a photon collides with an electron - the photon will either give up energy to or gain energy from the electron, changing the electron's velocity as a result. |
What Are Some Examples of Continuum Emission?
Gas that is hotter than 10 million degrees, such as the gas heated by a supernova explosion, produces most of its emission in X-rays from thermal Bremsstrahlung. Gas can be heated to these temperatures by the outward moving shock of a supernova explosion, or in an accretion disk around a black hole or neutron star. Synchrotron radiation can produce X-rays around supernova remnants (SNR), where the magnetic fields are strong and ions have been accelerated by the shock wave to high energies. X-rays produced by SNR require electrons with energies of about 104 GeV (Giga electron-Volts) each (you would have to heat an electron to a temperature of about ten trillion degrees for it to have this much energy)! Synchrotron radiation and Compton scattered radiation are major components of the diffuse X-ray background and emission from active galaxies.
For the StudentUsing the text, define the following terms: spectroscopy, keV, continuum, continuum emission, line emission, electromagnetic spectrum, synchrotron radiation, bremmstrahlung, comptonization. |
Reference URLs:
Spectroscopy
http://imagine.gsfc.nasa.gov/science/toolbox/spectra1.html
http://www.colorado.edu/physics/PhysicsInitiative/Physics2000/quantumzone/
Back to the Main Spectra Unit Menu

